Abstract
Introduction
Tenalisib (RP6530) is a next generation, oral, selective, PI3Kδ/g inhibitor with nanomolar inhibitory potency. Besides its apoptotic and anti-proliferative activity, Tenalisib modulates the tumor microenvironment resulting in reprogramming of tumor associated-macrophages (TAMs) from a protumor M2 phenotype to an antitumor M1 phenotype and a marked reduction of angiogenesis in pre-clinical models. Tenalisib has demonstrated activity in patients with relapsed/refractory lymphoid malignancies (Carmelo, ASH 2016 and Oki, ASCO 2018).
Since there are concerns over long-term safety of PI3K δ or PI3K dual δ/g inhibitors with respect to immune-mediated toxicities (e.g. transaminitis, colitis and pneumonitis), cytopenias, and infections, a pooled safety analysis across two Phase I studies in patients treated with Tenalisib was performed.
Methods
Safety data was pooled from two Phase I Tenalisib monotherapy trials (NCT02017613 and NCT02567656) with similar key eligibility criteria. Patients had R/R lymphoid malignancies with ≥1 prior therapy. Responses were evaluated in lymphoid malignancies using IWG criteria (Cheson et al., 2007) and in CTCL using the modified Severity Weighted Assessment Tool (mSWAT). Adverse events were graded according to CTCAE v4.03.
Results
A total of 93 patients were included in the analysis. Among these patients, 34% were PTCL, 32% CTCL, 16% HL, 6% DLBCL and 12% were other lymphomas. Patients received a median of 5 prior therapies. 53 % of patients received Tenalisib for ≤ 3 months, 21% for 3-6 months and 26% for > 6 months.
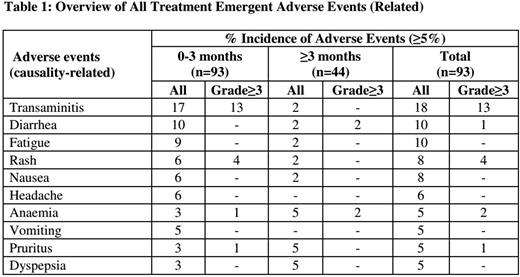
The overall incidence of related AEs and ≥G3 AEs were 58% and 29% respectively (Table 1). Very few AEs were seen with exposures >6 months and mainly included single cases of diarrhea, anemia, edema, and abdominal pain. There were no incidences of late onset toxicities such as colitis and pneumonitis and most of the AEs happened during the initial three months of therapy. No treatment discontinuations due to AE's were seen in patients exposed to > 6 months of treatment.
Efficacy response assessments of the 66 evaluable patients demonstrated an ORR of 45% in TCL (44% in PTCL (8/18, 3 CR, 4 PR) & 45% in CTCL (9/20, 9 PR)), 29% (4/14; 1CR; 3PR) in HL, and 13% (1/6; 1CR) in DLBCL.
Conclusion
In this pooled safety analysis with long term follow-up, Tenalisib exhibited an improved safety profile when compared to other investigational/marketed PI3K inhibitors. The incidence of transaminitis was low and occurred within the first two to three cycles of therapy. In particular, there were no occurrences of pneumonitis or colitis in patients that had been on treatment for > 3 months and beyond. Incidence of neutropenia/thrombocytopenia and infections was limited. Tenalisib can therefore be safely combined with a diverse array of other agents active in lymphoid malignancies. Tenalisib is currently being studied in combination with Pembrolizumab and Romidepsin and as a monotherapy in a Phase II trial in indolent NHL.
Haverkos:Viracta Therapeutics: Membership on an entity's Board of Directors or advisory committees. Ramchandren:Pharmacyclics LLC an AbbVie Company: Consultancy, Research Funding; Seattle Genetics: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy; Merck: Research Funding; Janssen: Consultancy, Research Funding. Devata:Affimed: Research Funding. Routhu:Rhizen Pharmaceuticals SA: Employment. Barde:Rhizen Pharmaceuticals SA: Employment. Nair:Rhizen Pharmaceuticals SA: Employment. Carlo-Stella:Boehringher Ingelheim Italia: Consultancy; Sanofi: Consultancy; MSD Italia: Speakers Bureau; Rhizen Pharmaceuticals: Research Funding; AstraZeneca: Speakers Bureau; ADC Therapeutics: Research Funding, Speakers Bureau; Janssen: Speakers Bureau; Genenta Science: Speakers Bureau; Bristol-Myers Squibb: Speakers Bureau; Amgen: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal